What are the latest proposals for DSM-5 “Somatic Symptom Disorders” categories and why are they problematic? (Part 1)
Post #75 Shortlink: http://wp.me/pKrrB-12P
DSM stands for Diagnostic and Statistical Manual of Mental Disorders. The DSM is published by the American Psychiatric Association (APA) and contains descriptions, symptoms and criteria for diagnosing mental disorders. It does not include information or guidelines on treatments. DSM is the primary diagnostic system in the US for defining mental disorders and is used to a varying extent in other countries.
As a classification system, DSM does not have quite the significance in the UK as Chapter V: Mental and Behavioural Disorders of the WHO’s ICD-10, which is used more often in Europe for classifying mental health disorders. But the next edition of DSM will shape international research, influence literature in the fields of psychiatry and psychosomatics and inform health care providers and policy makers’ perceptions of patients’ needs for many years to come.
The next edition of DSM, which will be known as DSM-5, is scheduled for publication in May 2013.
Diagnostic criteria defined within the DSM determine what is considered a mental disorder and what is not, what medical treatments individuals receive and which treatments medical insurers will authorise funding for. In addition to use in medical settings, DSM is also used by social services agencies, governments, policy makers, courts, prisons, drug regulation agencies, pharmaceutical companies and in research.
The inclusion or not of a disorder within DSM has revenue implications for pharmaceutical companies seeking licences for new drugs or to expand markets and applications for existing products.
Second public review of proposals for DSM-5
On 4 May, the APA published revised proposals for the 13 Work Groups for the revision of DSM-IV categories and diagnostic criteria on the DSM-5 Development website and issued a news release announcing a second stakeholder review and feedback exercise. According to the DSM-5 Timeline, as it stood in March, this second public review was not expected until August-September.
Q: Is this review and comment process open only to APA members and other professionals?
A: No. All stakeholders are invited to submit comment and feedback on the draft framework and the latest proposed revisions to diagnostic criteria: patients and families, patient advocates and patient representation organizations as well as clinicians, researchers, allied health professionals, lawyers and other end users.
Q: How long will this second review period run for?
A: The DSM-5 Development website is open for commenting now until 15 June.
Q: Is registration required in order to submit feedback?
A: Yes. You will need to register to submit comment to the Work Groups. You can register now on the DSM-5 Development site to participate. Once registered, you can prepare and upload your comment via a WYSIWYG editor anytime until 15 June. More information on registering to submit feedback in Post #78.
Q: Which DSM-5 Work Group proposals have potentially the most implications for CFS and ME patients?
A: The DSM-5 Work Group which has the most relevance for “Chronic fatigue syndrome”, CFS, “ME”, “CFS/ME”, “ME/CFS”, IBS, Fibromyalgia, Chemical Sensitivity (CS), Chemical Injury (CI), Environmental Illness (EI), GWS and chronic Lyme disease patients is the Somatic Symptom Disorders Work Group (SSD Work Group) which has responsibility for the revision of the categories currently classified in DSM-IV under “Somatoform Disorders”.
Q: Where can I find copies of the comments submitted last year by ME and CFS patient organizations during the first public review?
A: Copies of comments submitted, last year, by international patient organizations to the Work Group for “Somatic Symptom Disorders” are collated here together with some of the feedback submitted by patients and patient advocates: http://tinyurl.com/DSM5submissions
Q: How many submissions did the 13 DSM-5 Work Groups and Task Force receive during the first review?
A: The APA reports having received over 8000 comments across all categories. After the review period had closed, the Task Force did not publish summaries of key areas of concern brought to its attention by stakeholders and neither has the Task Force nor individual Work Groups published responses to areas of major concern.
Q: How many submissions were received in response to the proposals published last year for the “Somatic Symptom Disorders” categories?
A: The APA did not publish a breakdown of the numbers of responses received by each of the 13 Work Groups.
Q: How do the current DSM-IV categories for “Somatoform Disorders” compare with ICD-10?
A: There is a degree of correspondence between the current Somatoform Disorders section in DSM-IV and the equivalent section in ICD-10 Chapter V Mental and behavioural disorders. This simplified table sets out how the two classification systems currently correspond for their respective Somatoform Disorders categories:
Current DSM-IV Codes and Categories for Somatoform Disorders and ICD-10 Equivalents
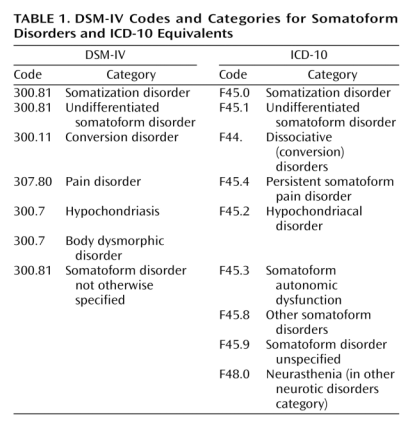
Source: Mayou R, Kirmayer LJ, Simon G, Kroenke K, Sharpe M: Somatoform disorders: time for a new approach in DSM-V. Am J Psychiat. 2005;162:847–855.
Neurasthenia is not categorized in DSM-IV. Neurasthenia is classified in ICD-10 in Chapter V Mental and behavioural disorders, at F48.0, as shown in the table, above.
Chronic fatigue syndrome is not classified in DSM-IV. Chronic fatigue syndrome is indexed in ICD-10 to G93.3, (Chapter VI Diseases of the nervous system – the Neurology chapter), the same code to which PVFS and (Benign) ME are classified.
ICD-10 has “Fatigue syndrome” [Note: not “postviral”; not “chronic”] coded at F48.0 in Chapter V, which specifically excludes G93.3 Postviral fatigue syndrome.
(Please refer to the “ICD-10 Classification of Mental and Behavioural Disorders Clinical descriptions and diagnostic guidelines” aka “the Blue Book” and to ICD-10 online for full categories, disorder descriptions, inclusions and exclusions for ICD-10 Somatoform Disorders.) [9] [10]
Q: What does “Harmonization” between DSM-IV and the forthcoming ICD-11 mean?
A: The APA participates with the WHO in an International Advisory Group for the Revision of ICD-10 Mental and Behavioural Disorders and a DSM-ICD Harmonization Coordination Group.
There is already a degree of correspondence between some categories in DSM-IV and their equivalent sections in ICD-10 Chapter V. For their next editions, the APA and the WHO have committed as far as possible:
“To facilitate the achievement of the highest possible extent of uniformity and harmonization between ICD-11 mental and behavioural disorders and DSM-V disorders and their diagnostic criteria.”
with the objective that
“The WHO and APA should make all attempts to ensure that in their core versions, the category names, glossary descriptions and criteria are identical for ICD and DSM.”
The WHO acknowledges there may be areas where congruency between the two systems may not be achievable.
Q: Is ICD Revision intending to harmonize its Somatoform Disorders categories with the current proposals for DSM-5?
A: DSM-5 proposals are a “work in progress”. The proposals of the Somatic Symptom Disorders Work Group for the revision of categories within this section represent a radical restructuring of the current DSM-IV Somatoform Disorders; following fields trials, the Work Group will review and potentially revise their proposals. These proposals may be found to be inoperable in the field or otherwise unacceptable to clinicians participating in field trials. The Task Force may require the Work Group to make substantial modifications to the current proposals. A third public review is scheduled for January-February 2011 prior to the finalization of categories and criteria.
It’s not known how closely the DSM-5 Work Group for “Somatic Symptom Disorders” are collaborating with the ICD Revision working group responsible for overseeing the revision of ICD-10’s Somatoform Disorders categories.
There have been no minutes or summaries of meetings of the International Advisory Group for the Revision of ICD-10 Mental and behavioural disorders, in which the APA participates and which is chaired by DSM-5 Task Force member, Steven E Hyman, MD, published since December 2008 (a point raised recently with the WHO’s Dr Bedhiran Üstün) and the ICD Revision Topic Advisory Group for Mental Health does not issue public reports on its progress.
It is not known whether, to what extent or at what stage in the Alpha/Beta drafting process ICD Revision might seek to achieve congruency between category names, glossary descriptions and criteria for ICD-11 Chapter 5 and those being proposed for the restructured DSM “Somatoform Disorders” section. But the classifications under “Somatoform Disorders” for ICD-11 Chapter 5, according to the iCAT Alpha Drafting platform as it stood in November, last year, did not appear to mirror the proposals of the DSM-5 SSD Work Group:
Chapter 5 (V) Somatoform Disorders (the F codes) F45 – F48.0 (as displaying in the iCAT Alpha Drafting platform in November 2010):

(It is understood from ICD documentation that the child categories F45.40 and F45.41 are proposed new entities for ICD-11.)
From what is understood of ICD taxonomic and ontological principles, the conceptual framework and radical restructuring of the Somatoform Disorders currently proposed by the SSD Work Group, might prove difficult for ICD-11 to assimilate even if ICD Revision were to consider the proposals, per se, to be valid constructs that could be used reliably.
Q: What proposals are being put forward for the revision of the DSM-IV categories currently known as “Somatoform Disorders”?
A: The SSD Work Group is recommending renaming the “Somatoform Disorders” disorders section of DSM-IV to “Somatic Symptom Disorders”.
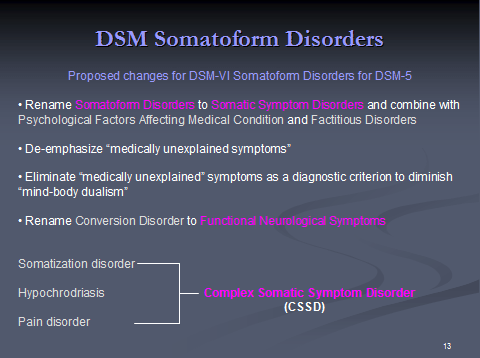
The Work Group proposes combining existing categories – Somatoform Disorders, Psychological Factors Affecting Medical Condition (PFAMC), Factitious Disorder and Factitious Disorder imposed on another (previously known as Factitious Disorder by proxy) into one group entitled “Somatic Symptom Disorders”. Alternatively, Factitious Disorders would be listed under the category “Other Disorders”.
The Work Group’s summary justification is ‘Because the current terminology for somatoform disorders is confusing and because somatoform disorders, psychological factors affecting medical condition, and factitious disorders all involve presentation of physical symptoms and/or concern about medical illness, the work group suggests renaming this group of disorders Somatic Symptom Disorders. In addition, because of the implicit mind-body dualism and the unreliability of assessments of “medically unexplained symptoms,” these symptoms are no longer emphasized as core features of many of these disorders.’
‘…since Somatization Disorder, Hypochondriasis, Undifferentiated Somatoform Disorder, and Pain Disorder share certain common features, namely somatic symptoms and cognitive distortions, the work group is proposing that these disorders be grouped under a common rubric under a new category called “Complex Somatic Symptom Disorder” (CSSD).’
There is a relatively recent additional proposal for a category called “Simple (or abridged) Somatic Symptom Disorder” (SSSD).
These proposals would represent a major change in the diagnostic nomenclature for this section of the DSM.
The Work Group also proposes a category “Illness Anxiety Disorder” (hypochondriasis without somatic symptoms) and recommends the existing Conversion Disorder category be renamed “Functional Neurological Disorder”. (‘Somatic’ means of or relating to the body.)
Q: Have there been changes since the publication of the initial proposals, in February 2010?
A: Since the first public review, the Work Group has modified the criteria for “Complex Somatic Symptom Disorder (CSSD), added a new proposal for a category called “Simple Somatic Symptom Disorder” and made revisions to the text of the two key PDF documents. So you will need to review the most recent criteria and the two key documents that accompany these latest proposals if you are intending to submit comment.
I shall be posting the latest proposals for criteria and the two key “Disorder Description” and “Rationale” documents in the next post (Post #77).
References
1] APA 4 May 2011 News release No. 11-27 or http://tinyurl.com/APAnewsrelease4may11
2] “Somatic Symptom Disorders” Work Group Members, Bios and Disclosures
3] Latest proposals for “Somatic Symptom Disorders”
4] Key Somatic Symptom Disorders PDF Document: Disorder Descriptions
5] Key Somatic Symptom Disorders PDF Document: Justification of Criteria
6] Revised DSM-5 Timeline
7] Register on the DSM-5 site to submit stakeholder feedback
8] APA’s FAQ on DSM-5
[9] ICD-10 online (version for 2007) Chapter V: Somatoform Disorders: F45-F48.0 codes”
[10] ICD-10 Classification of Mental and Behavioural Disorders Clinical descriptions and diagnostic guidelines” (aka “the Blue Book”) PDF format
Disorders Description Key Document One: “Somatic Symptom Disorders”
Rationale Document Key Document Two: “Justification of Criteria — Somatic Symptoms”



