October 26, 2010
by meagenda
APA Announces Start of Field Trials for DSM-5; MedPage Today commentary
Post #50 Shortlink: http://wp.me/pKrrB-QC
APA News Release
PDF: News Release 05.10.10
American Psychiatric Association (APA)
For Information Contact:
Eve Herold, 703-907-8640
press@psych.org
Jaime Valora, 703-907-8562
jvalora@psych.org
For Immediate Release:
Oct. 5, 2010
Release No. 10-65
APA Announces Start of Field Trials for DSM-5
Sites to Test Proposed Diagnostic Criteria in Real-World Clinical Settings
ARLINGTON, Va. (Oct. 5, 2010) – The American Psychiatric Association today announced the start of field trials to test proposed diagnostic criteria for the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Field trials will help assess the practical use of proposed DSM-5 criteria in real-world clinical settings.
The field trials follow a public comment period in which more than 8,000 written comments on the draft diagnostic criteria were submitted to the DSM-5 website by clinicians, researchers and family and patient advocates. Submitted comments were reviewed by DSM-5 Work Groups and resulted in further refinement of the criteria.
Evaluation measures
For the diagnostic criteria that are being evaluated, the results of the field trials will address:
. Feasibility: are the proposed criteria easy for clinicians to understand and to use?
. Clinical Utility: do the proposed criteria do a good job in describing patients’ psychiatric problems and help clinicians make decisions about treatment plans?
. Reliability: are the same conclusions reached consistently when the criteria are used by different clinicians?
. Validity: how accurately do the diagnostic criteria reflect the mental disorders they are designed to describe?
In addition, the field trials will help assess severity measures and cross-cutting dimensional measures.
Severity measures are questionnaires and other tools intended to help clinicians evaluate how severe the symptoms of an individual are on a rating scale.
Cross-cutting dimensional measures are tools for assessing symptoms that occur across a wide range of diagnoses, such as anxiety or sleep problems. Field trials will help determine whether these proposed tools provide useful information for clinicians and their patients, and whether they capture changes in symptoms over time to evaluate progress in treatment.
Two rigorous study designs
Since the DSM is used in many care settings, two standardized and methodologically rigorous study designs were developed by the DSM-5 Research Group to gather data from a wide range of clinicians and settings.
“It is important that the proposed diagnostic criteria are subjected to rigorous and empirically sound field trials before DSM-5 is published in 2013,” said David Kupfer, M.D., chair of the DSM-5 Task Force.
“The two field trial designs will allow us to better understand how the proposed revisions affect clinicians’ practices and, most importantly, patient care.”
One study design was developed for use in academic or other large clinical settings, and will be employed at 11 sites, chosen from among 65 centers that responded to APA’s call for proposals. Another study design was developed for use by individual practitioners and smaller clinical practices. These field trials will be conducted in diverse care settings by 3,900 mental health professionals: 1,400 psychiatrists selected from a randomly selected sample, as well as an additional 2,500 volunteer clinicians, including psychiatrists, psychologists, social workers, and advanced practice psychiatric-mental health nurses.
Participating clinicians must meet eligibility criteria and complete a web-based training seminar.
Clinicians in the field trials will evaluate new and existing patients at different stages of treatment using the proposed DSM-5 diagnostic criteria and measures.
All patients considered for participation in the field trial will receive information about the trial and must give their consent. None of the patients will have their identities revealed in the results of the studies.
In the field trials conducted in the academic and large medical centers, patient evaluations will begin with an initial baseline assessment by a clinician. A different clinician will conduct a second assessment 4 hours to 2 weeks later, to help determine reliability of the diagnostic criteria. This assessment will be repeated in a follow-up visit (4 to 12 weeks after the second evaluation) to test whether the severity and cross-cutting measures are sensitive to changes in treatment progression.
Academic and Large Medical Centers
The 11 large academic medical settings participating in field trials are:
Pediatric Sites
. Baystate Medical Center, Springfield, Mass.
. Columbia University/New York State Psychiatric Institute, Child Psychiatry Division, in collaboration with colleagues at New York Presbyterian
. Hospital/Weill Cornell Medical Center, New York Presbyterian
. Hospital/Westchester Division, and the North Shore Child and Family Guidance Center, Roslyn Heights, New York
. Stanford University, Lucile Packard Children’s Hospital, Palo Alto, Calif.
. The Children’s Hospital, Aurora, Colo.
Adult Sites
. Centre for Addiction and Mental Health, Toronto
. Dallas Veterans Affairs Medical Center
. DeBakey Veterans Affairs Medical Center and Menninger Clinic, Baylor College of Medicine, Houston
. Mayo Clinic, Rochester, Minn.
. University of California, Los Angeles
. University of Pennsylvania, Philadelphia
. University of Texas Health Science Center, San Antonio
More information on the participating academic large medical centers and the specific disorders being tested in field trials is available on www.dsm5.org .
Disseminating the Field Trial Findings
The DSM-5 Field Trials team will disseminate the results of these initial field trials through presentations at scientific meetings, with professional and consumer groups and in articles published in peer-reviewed scientific journals and DSM-5 source books.
After completion of the first phase of field trials and another period of public comment via the DSM5.org web site, work group members will make any necessary revisions to their draft criteria. This will be followed by a second phase of field trials for further examination of selected criteria, scheduled to take place in 2011 and 2012.
“The process for developing DSM-5 continues to be deliberative, thoughtful and inclusive,” said Darrel Regier, M.D., M.P.H., vice-chair of the DSM-5 Task Force, and APA research director. “Large-scale field trials are the next critical phase in this important process and will give us the information we need to ensure the diagnostic criteria are both useful and accurate in real-world clinical settings.”
The American Psychiatric Association is a national medical specialty society whose physician members specialize in the diagnosis, treatment, prevention and research of mental illnesses, including substance use disorders.
Visit the APA at http://www.psych.org and www.healthyminds.org .
[Ends]
Commentary and previous commentaries on the development of DSM-5 from MedPage Today here:
http://www.medpagetoday.com/Psychiatry/DSM-5/
http://www.medpagetoday.com/Psychiatry/DSM-5/22579
DSM-5 Field Trials Off to Late Start
By John Gever, Senior Editor, MedPage Today
Published: October 05, 2010
“Testing of new diagnostic criteria proposed for DSM-5, the revision of the psychiatric profession’s manual for patient assessment, is finally underway, more than two months behind schedule…”
(With thanks to Kelly Latta for alerting me to the MedPage Today commentary.)
———-
Current proposals by the DSM-5 Work Group for disorders related to the diagnostic category, Somatoform Disorders, can be found here:
http://www.dsm5.org/ProposedRevisions/Pages/SomatoformDisorders.aspx
and here, in Post #17, on Dx Revision Watch site:
Proposed revisions and draft criteria for DSM-5 categories were published by the American Psychiatric Association on 10 February
DSM-5 Submissions to the public review process
There were considerable concerns, earlier this year, in response to the proposal of the DSM-5 Work Group for “Somatic Symptom Disorders” to combine several existing somatoform disorder categories into one larger category, Complex Somatic Symptom Disorder (CSSD).
Patient organisations, professionals and advocates submitting comments in the DSM-5 draft proposal public review process were invited to provide copies of their submissions for publication on this page:
http://wp.me/PKrrB-AQ
———-
This table sets out how the current versions of classification systems, DSM-IV and ICD-10, have corresponded for Somatoform Disorders:
Current DSM-IV Codes and Categories for Somatoform Disorders and ICD-10 Equivalents
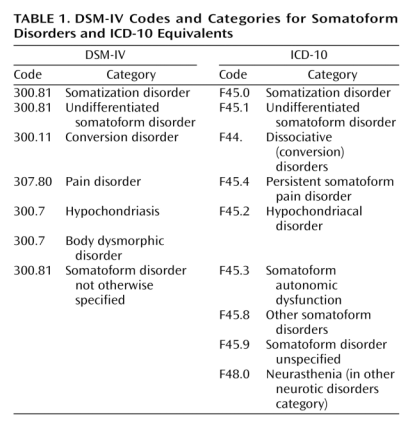
Source: Mayou R, Kirmayer LJ, Simon G, Kroenke K, Sharpe M: Somatoform disorders: time for a new approach in DSM-V. Am J Psychiat. 2005;162:847-855.
ICD-11 Alpha Draft
According to sources, in July, a print version of the ICD-11 Alpha Draft was expected to be made available around the time that the rescheduled iCamp2 meeting took place in September.
In August, ICD Revision confirmed that a “draft print version will be available in September 2010”.
iCamp2 has now concluded, but it remains unclear whether a print version has been produced. ICD Revision has been asked to clarify the status and availability of an Alpha Draft, whether it is intended for internal use only or is going to be made available for public scrutiny, and if so, when, and in what format(s).
For update on status and availability of ICD-11Alpha Draft see: Post #53
The publication of DSM-5 is currently timelined for May 2013.
Implementation of ICD-10-CM, the US specific “Clinical Modification” of ICD-10, is scheduled for October 2013.
According to the APA’s DSM-5 website Timeline:
http://www.dsm5.org/about/Pages/Timeline.aspx
[…]
As the Phase 1 field trials are underway, members of the DSM-5 Task Force and Work Group will begin drafting their initial text for DSM-5. During this time, case studies will also be developed, which will be published after DSM-5’s release in a series of case books.
March – April 2011: Revisions to Proposed Criteria. Based on results from the first phase of field trials, the DSM-5 Task Force and Work Group members will make revisions to the proposed DSM-5 diagnostic criteria and dimensional measures. These revised criteria and measures will be tested in a second phase of field trials.
April – May 2011: Review of Revised Criteria. Revised proposed criteria will be subjected to internal review, including a review by the DSM-5 Task Force and Research Group and by other relevant work groups.
May-July 2011: Online Posting of Revised Criteria. Following the internal review, revised draft diagnostic criteria will be posted online for approximately one month to allow the public to provide feedback. This site will be closed for feedback by midnight on June 30, 2011.
[…]

