June 21, 2012
by meagenda
Three professional organization responses to the third and final DSM-5 stakeholder review
Post #185 Shortlink: http://wp.me/pKrrB-2hS
According to DSM-5 Task Force Vice-chair, Darrel Regier M.D., the specific diagnostic categories that received most comments during the second public review of draft proposals (May-June 2011) were the sexual and gender identity disorders, followed closely by somatic symptom disorders and anxiety disorders.
The American Psychiatric Association (APA) has yet to report how many comments the DSM-5 Task Force and its 13 Work Groups received during this third and final review period (which closed last Friday), or which categories garnered the most responses, this year.
No publication of field trial data
Following posting of the third draft on May 2, it was anticipated APA would publish full results from the DSM-5 field trials “within a month”. [Source: Deborah Brauser for Medscape Medical News: interview with Darrel Regier, May 8, 2012.]
No report emerged and stakeholders had little choice but submit feedback on this latest iteration without the benefit of scrutiny of reliability data to inform their submissions.
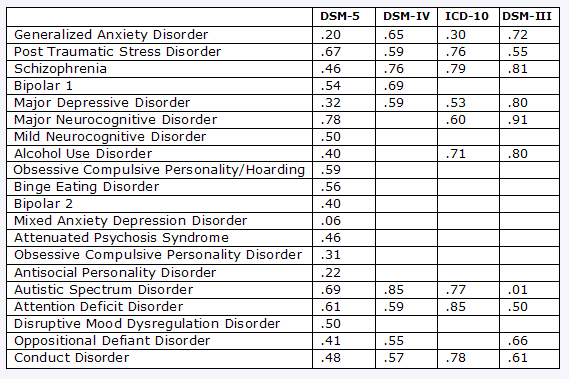
APA has yet to account for its failure to place its field trial results in the public domain while the feedback exercise was in progress, other than releasing some Kappa data at its May 5-9 Annual Conference.
American Psychiatric Association CEO and Medical Director, James H. Scully, Jr., M.D., blogs at Huffington Post. Last week, I asked Dr Scully why the field trial report has been withheld; whether Task Force still intends publishing field trial data and when that report might now be anticipated.
I’ve received no response from Dr Scully and APA has put out no clarification.
No publication of list of Written Submissions
These three DSM-5 public reviews of draft proposals for changes to DSM-IV categories and criteria have not been managed as formal stakeholder consultation exercises.
APA publishes no aggregations of key areas of concern identified during public comment periods nor publishes Work Group or Task Force responses to key areas of professional or lay public concern on the DSM-5 Development website – an issue I raised with the Task Force during both the first and second reviews.
Although some published submissions (ACA, British Psychological Society and the DSM-5 Reform Open Letter and Petition Committee) have received responses from the Task Force and which APA has elected to place in the public domain, submissions from the majority of professional bodies and organizations disappear into a black hole.
In the interests of transparency, APA could usefully publish lists of the names of US and international professional bodies, academic institutions, patient advocacy organizations etc. that have submitted comments, in the way that Written Submissions are listed in the annexes to reports and public inquiries.
That way, interested parties might at least approach organizations to request copies of submissions or suggest that these are placed in the public domain.
APA could not legitimately claim it would require permissions before publishing full lists of the names of professional body, academic institution and organization respondents that tendered formal responses – its legal department’s boilerplate Terms and Conditions of Use gives APA carte blanche to make use of and publish uploaded submissions in any way it sees fit.*
*See Terms and Conditions of Use, under “User Submissions”
The following have released their submissions in response to the third draft:
Submission from The American Mental Health Counselors Association (AMHCA)
The American Mental Health Counselors Association is a nationwide organization representing 6,000 clinical mental health counselors. Their submission includes concerns for the lowering of the “B type” threshold requirement for “Somatic Symptom Disorder” criteria between the second and third drafts.
[In the CSSD field trials, about 15% of the “diagnosed illness” study group (patients with cancer and coronary disease) met the criteria for coding with an additional mental health diagnosis of “SSD” when “one B type” cognition was required; about 10% met the criteria when “two B type” were required. About 26% of the “functional somatic” arm of the study group (patients with irritable bowel and “chronic widespread pain” – a term used synonymously with fibromyalgia) met the criteria for coding with an additional mental health diagnosis of “SSD” when “one B type” cognition was required; about 13% met the criteria when “two B type” were required. AMHCA recommends raising the threshold back to at least two from the three B type criteria, as the criteria for CSSD had stood for the second draft. I consider the category of “SSD” should be rejected in the absence of a substantial body of independent evidence for the reliability, validity and safety of “SSD” as a construct.]
AMHCA Submits Comments on DSM-5 06/19/12
June 18, 2012 – Alexandria, VA – The DSM-5 Task Force of the American Mental Health Counselors Association (AMHCA) has submitted comments for the third period of public comment on the fifth edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-5).
AMHCA’s comments addressed 12 disorder categories and the Cultural Formulation Interview Guide. Per the site requirements, each was sent separately to the particular disorder site.
 Download compilation of comments submitted by AMHCA DSM-5 Task Force
Download compilation of comments submitted by AMHCA DSM-5 Task Force
“Somatic Symptom Disorders
“Somatic Symptom Disorder
“A major change in this revision is the merger of Complex Somatic Symptom Disorder and Simple Somatic Symptom Disorder into one disorder, Somatic Symptom Disorder. The increased emphasis placed on cognitive distortions (along with the presence of somatic symptoms ) provides greater clarity about the nature of the disorder. However, the notion that a single B.2 criteria could be used as the sole basis for identifying these cognitive aspects seems to open the door to diagnosing individuals who have legitimate “high anxiety” about their symptoms. We recommend considering “two of three” criteria under B be required.”
The British Psychological Society writes:
The British Psychological Society still has concerns over DSM-V
…For all the reasons stated above, the BPS, having reviewed the currently proposed revisions of the new diagnostic criteria in DSM 5, continues to have major concerns. These have, if anything, been increased by the very poor reliabilities achieved in many of the recent field trials (Huffington Post, 2012), especially given the limited time available to attempt to achieve more satisfactory outcomes. Since validity depends, at the very least, on acceptable levels of reliability, the unavoidable conclusion is that many of the most frequently-used categories will be unable to fulfil their purported purposes, i.e. identification of appropriate treatments, signposting to support, providing a basis for research…
Read full submission to third draft here in PDF format.
Response to second draft here.
Christopher Lane comments:
Psychology Today | Side Effects
Arguing Over DSM-5: The British Psychological Society Has Serious Concerns About the Manual
The BPS expresses “serious reservations” about the next DSM.
Christopher Lane, Ph.D. in Side Effects | June 20, 2012
Although the American Psychiatric Association recently closed its window allowing comments on proposed changes to the DSM, the organization has yet to report on the field trials it devised for the next edition of the psychiatric manual, themselves meant to support—indeed, serve as a rationale for—the changes it is proposing in the first place.
While this unhappy outcome points to some of the organization’s chicken-and-egg problems with the manual and the disorders it is seeking to adjust or make official, those wanting to respond to the draft proposals have had to do so in the dark, unaware of the results of the field trials and thus whether the proposals draw from them any actual empirical support…
Read on
Submission from American Counseling Association (ACA)
The American Counseling Association (ACA), represents more than 50,000 counselors – one of the largest groups of DSM-5 users in the US.
ACA provides final comments on the DSM-5
ACA President Don W. Locke has sent the American Psychiatric Association a letter providing final comments for the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Based on comments from ACA members and the ACA DSM Task Force, the letter acknowledges useful changes that had been made to previous drafts of the DSM-5: the development of the Cultural Formulation Outline, reversing the pathologizing of normal bereavement, and limiting the expansion of personality disorder types. ACA also calls for addressing the one-dimensional nature of the new Substance Use Disorder category and rejects the proposed dimensional assessments. Click here to view letter.
This is the third letter ACA has sent to the American Psychiatric Association providing feedback for the DSM-5. Click the links below to read the previous letters and a response from APA:
Letter from President Lynn Linde, April 16, 2010
Letter from President Don Locke, November 8, 2011
Response from APA President John Oldham, November 21, 2011
Submission by Coalition for DSM-5 Reform Committee
The Coalition for DSM-5 Reform Open Letter and Petition has garnered support from over 13,700 professionals and concerned stakeholders and the endorsement of nearly 50 organizations, since launching last October.
The DSM-5 Reform Committee continues to call for independent scientific review of draft proposals and submitted the following response during this third and final comment period:
Submission from Coalition for DSM-5 Reform (Society for Humanistic Psychology)Division 32 of the American Psychological Association)
To the DSM-5 Task Force and the American Psychiatric Association
As you know, the Open Letter Committee of the Society for Humanistic Psychology and the Coalition for DSM-5 Reform have been following the development of DSM-5 closely.
We appreciate the opportunity for public commentary on the most recent version of the DSM-5 draft proposals. We intend to submit this brief letter via the dsm5.org feedback portal and to post it for public viewing on our website at http://dsm5-reform.com/
Since its posting in October 2011, the Open Letter to the DSM-5, which was written in response to the second version of the draft proposals, has garnered support from almost 50 mental health organizations and over 13,500 individual mental health professionals and others.
Our three primary concerns in the letter were as follows: the DSM-5 proposals appear to lower diagnostic thresholds, expanding the purview of mental disorder to include normative reactions to life events; some new proposals (e.g., “Disruptive Mood Dysregulation Disorder” and “Attenuated Psychosis Syndrome”) seem to lack the empirical grounding necessary for inclusion in a scientific taxonomy; newly proposed disorders are particularly likely to be diagnosed in vulnerable populations, such as children and the elderly, for whom the over-prescription of powerful psychiatric drugs is already a growing nationwide problem; and the increased emphasis on medico-biological theories for mental disorder despite the fact that recent research strongly points to multifactorial etiologies.
We appreciate some of the changes made in this third version of the draft proposals, in particular the relegation of Attenuated Psychosis Syndrome and Mixed Anxiety-Depression to the Appendix for further research. We believe these disorders had insufficient empirical backing for inclusion in the manual itself. In addition, given the continuing elusiveness of biomarkers, we are relieved to find that you have proposed a modified definition of mental disorder that does not include the phrase “underlying psychobiological dysfunction.”
Despite these positive changes, we remain concerned about a number of the DSM-5 proposals, as well as the apparent setbacks in the development process.
Our continuing concerns are:
• The proposal to include new disorders with relatively little empirical support and/or research literature that is relatively recent (e.g., Disruptive Mood Dysregulation Disorder)
• The lowering of diagnostic thresholds, which may result in diagnostic expansion and various iatrogenic hazards, such as inappropriate treatment and stigmatization of normative life processes. Examples include the newly proposed Minor Neurocognitive Disorder, as well as proposed changes to Generalized Anxiety Disorder, Attention Deficit/Hyperactivity Disorder, Pedophilia, and the new behavioral addictions.
• The perplexing Personality Disorders overhaul, which is an unnecessarily complex and idiosyncratic system that is likely to have little clinical utility in everyday practice.
• The development of novel scales (e.g., severity scales) with little psychometric testing rather than utilizing established standards.
In addition, we are increasingly concerned about several aspects of the development process. These are:
• Continuing delays, particularly in the drafting and field testing of the proposals.
• The substandard results of the first set of field trials, which revealed kappas below accepted reliability standards.
• The cancelation of the second set of field trials.
• The lack of formal forensic review.
• Ad hominem responses to critics.
• The hiring of a PR firm to influence the interpretation and dissemination of information about DSM-5, which is not standard scientific practice.
We understand that there have been recent attempts to locate a “middle ground” between the DSM-5 proposals and DSM-5 criticism. We believe that, given the extremity and idiosyncrasy of some of the proposed changes to the manual, this claim of a “middle ground” is more rhetorical and polemic than empirical or measured. A true middle ground, we believe, would draw on medical ethics and scientific standards to revise the proposals in a careful way that prioritizes patient safety, especially protection against unnecessary treatment, above institutional needs.
Therefore, we would like to reiterate our call for an independent scientific review of the manual by professionals whose relationship to the DSM-5 Task Force and/or American Psychiatric Association does not constitute a conflict of interest.
As the deadline for the future manual approaches, we urge the DSM-5 Task Force and all concerned mental health professionals to examine the proposed manual with scientific and expert scrutiny.
It is not only our professional standards, but also – and most importantly – patient care that is at stake. We thank you for your time and serious consideration of our concerns, and we hope that you will continue to engage in dialogue with those calling for reform of DSM-5.
Sincerely,
The DSM-5 Open Letter Committee of the Society for Humanistic Society, Division 32 of the American Psychological Association