World Health Assembly adopts ICD-11: When will member states start using the new edition?
June 17, 2019
Post #354 Shortlink: https://wp.me/pKrrB-4Sm
On May 25, 2019, the 72nd World Health Assembly voted unanimously to adopt the ICD-11, the next edition of the International Classification of Diseases (ICD).
Endorsement won’t come into effect until January 1, 2022, which is the earliest date that member states can begin using ICD-11 for reporting data.
A stable version of the ICD-11 MMS was released in June 2018 to enable member states to begin planning for implementation. This release was replaced in April 2019 with ICD-11 MMS Version: 04/2019.
ICD-11 is an electronic classification containing over 55,000 codes and a considerably more complex product than ICD-10. It has been designed to incorporate or link with other ICD classifications, such as the International Classification of Functioning, Disability and Health (ICF), the WONCA* developed International Classification of Primary Care (ICPC), and with the SNOMED-CT and OrphaNet terminologies.
Even the earliest implementers will need several years to evaluate the new edition, determine how they will use ICD-11, complete translations, produce training and implementation materials and prepare their health systems for migration. Japan is understood to be well advanced with translations and planning.
There is no mandatory implementation date: member states will migrate to ICD-11 at their own pace and according to their countries’ needs and resources but there is an expectation that countries will start planning for transition. Some member states may need to develop clinical modifications of ICD-11 for country specific use. A few countries still use ICD-9.
Global implementation of the new edition will be a patchy and prolonged process and during the transition period, WHO will be accepting data reported using both ICD-10 and the new ICD-11 code sets until the majority of member states have transitioned to the new edition. WHO has said that the last update to ICD-10 will be Version 2019.
No member states have announced timeline projections but below is a round-up of ICD-11 transition planning activities already in progress:
*World Organization of National Colleges, Academies and Academic Associations of General Practitioners/Family Physicians.
NHS England
NHS England mandates the use of ICD-10 in secondary care (currently using ICD-10 Version 2015).
As a WHO Collaborating Centre and designated UK Field Trial Centre, NHS Digital has taken part in ICD-11 Field Trials.
NHS Digital has said:
No decision has been made for the implementation of ICD-11 in England, however NHS Digital plan to undertake further testing of the latest release and supporting products that will inform a future decision.
NHS Digital Delen: ICD-11 resources page
Proposed Future Additions
Over the coming months, NHS Digital would like to engage and invite all users of ICD to participate and interact with the review process.
To support this, we are proposing to add the following information to our Delen site;
- A mechanism for questions, issues, concerns and errors relating to ICD-11 to be raised to us as the UK Field Trial Centre.
- A high-level overview of our future plans
- Presentations providing more information on ICD-11
- e-Learning materials to support familiarisation with ICD-11. Topics to include post coordination / cluster coding, chapter and code structure, chapter specific changes and notes, conventions etc
- Further testing – parallel coding in ICD-10 in real-time. If you would be interested in taking part in this please let us know by emailing icd-11@nhs.net
Until NHS England has implemented ICD-11, the mandatory classification system for use in the NHS remains ICD-10.
Since April 2018, SNOMED CT (which replaces the Read Codes/CTV3 clinical terminology) has been the mandatory terminology system for use in NHS primary care at the point of contact and forms an integral part of the electronic patient record (EPR).
SNOMED CT terminology system is already used in some secondary care settings but is planned to be implemented across all secondary care, acute care, mental health, community systems, dentistry and other systems used in direct patient care by April 2020.
SNOMED CT terminology system and clinical classifications, like ICD-10, work together to fulfil different needs:
Source: Presentation: NHS Digital: Clinical Coding for non coders – Overview of clinical coding, how ICD-10 and SNOMED CT work together, and the role of the Clinical Classifications Service.
For more information on the planning that will be required before ICD-11 can be implemented within the NHS, see BETA – Clinical Information Standards, section: ICD-11 and the new Procedure Based Classification (PBC).
Resources:
NHS Digital SNOMED CT resources
Australia
Australia uses a modification of the WHO’s ICD-10, known as ICD-10-AM [1].
Australian classification standards and statistics agencies were well represented on the ICD-11 Joint Task Force, with 5 of the Joint Task Force’s 21 members representing Australia, plus co-chair (Dr James Harrison, Director, Research Centre for Injury Studies, Flinders University, Adelaide) and observer (Dr Richard Madden, Professor of Health Statistics and Director National Centre for Classification in Health, University of Sydney).
For comparison, the UK had only an observer on the Joint Task Force; the U.S. had 4 participants and an observer.
The Australian Institute of Health and Welfare (AIHW) has been conducting a review of ICD-11 to inform and assist decision-makers about the new edition and its potential for adoption in Australia, see Post: #349: Australia: Potential adoption of ICD-11: Pre-consultation for decision makers.
1 Australian Consortium for Classification Development
Canada
Canada uses a modification of the WHO’s ICD-10, known as ICD-10-CA, developed by Canadian Institute for Health Information (CIHI) [1].
CIHI is participating in the testing of ICD-11 and assessing the implications for potential implementation in Canada.
CIHI has said that no decision has been made for the implementation of ICD-11 in Canada and that they are currently working on a number of initiatives to better understand the differences between ICD-10-CA and ICD-11 to help inform the business and statistical implications of adoption.
April 15, 2019 webinar:
https://www.cihi.ca/en/submit-data-and-view-standards/codes-and-classifications/icd-11
https://www.cihi.ca/fr/normes-et-soumission-de-donnees/codification-et-classification/cim-11
Introduction to ICD-11 — Part 1 Transcript and Recording
https://www.cihi.ca/en/bulletin/webinar-introduction-to-icd-11-part-1
https://www.cihi.ca/fr/bulletin/webinaire-introduction-a-la-cim-11-partie-1
Introduction to ICD-11 — Part 2 Transcript and Recording
https://www.cihi.ca/en/bulletin/webinar-introduction-to-icd-11-part-2
https://www.cihi.ca/fr/bulletin/webinaire-introduction-a-la-cim-11-partie-2
1 Version 2018 ICD-10-CA/CCI, Canadian Coding Standards and related products
United States
The National Center for Health Statistics (NCHS) is the federal agency responsible for the use of ICD-10 in the United States.
ICD-10 has been used in the U.S. to code and classify mortality data from death certificates since January 1999. NCHS developed a clinical modification of ICD-10 for morbidity purposes (ICD-10-CM) which replaced ICD-9-CM on October 1, 2015.
Since its initial launch, in 2007, the U.S. has maintained high level participation in the ICD-11 development process and its ongoing update and improvement:
The U.S. provided representatives from professional and scientific organisations, academics and practitioners for the ICD-11 Topic Advisory Groups (TAGs) and sub working groups. Stanford Center for Biomedical Informatics Research developed the web based iCAT Collaborative Authoring Platform on which ICD-11 was developed.
The U.S. has representatives on the ICD-11 governance committees via the WHO-FIC Network; the Medical Scientific Advisory Committee (MSAC); the Classifications and Statistics Advisory Committee (CSAC); the Mortality and Morbidity (MbRF) Reference Groups; and the Functioning and Disability Reference Group, which have oversight for the annual updating and ongoing improvement of the global ICD-11 edition.
Dr Geoffrey Reed (WHO, Geneva; Columbia University) is Senior Project Lead for the ICD-11 Mental Health chapter and a member of the MSAC; Steven Hyman, MD (former Director of the National Institute of Mental Health (NIMH) and former DSM-5 Task Force member) chaired the Topic Advisory Group for Mental Health; Michael B First, MD has served as a key external advisor to the Mental Health chapter. Harold Pincus, MD co-chaired the ICD-11 Quality and Patient Safety Topic Advisory Group.
Dr Christopher Chute (John Hopkins University) chaired the ICD-11 Revision Steering Committee, was a member of the Joint Task Force and now co-chairs the MSAC; Donna Pickett (Chief, Classifications and Public Health Data Standards, NCHS, Centers for Disease Control and Prevention, Head, Collaborating Center for the WHO-FIC in North America) co-chaired the Morbidity TAG, was a member of the Joint Task Force and is a member of the CSAC; Dr Robert Anderson (Chief, Mortality Statistics Branch Division of Vital Statistics, Centers for Disease Control and Prevention) was a member of the Joint Task Force and co-chaired the Mortality TAG; Cille Kennedy (ASPE) co-chaired the ICD-11 Functioning TAG; Sue Bowman (Senior Director of Coding Policy and Compliance, AHIMA) is a representative on the ICD-11 Morbidity Reference Group (MbRF).
Around 25 member states have modified ICD-10 for country specific use.
WHO is still formulating policies around the licensing of ICD-11 but it is understood that the intention is to limit development of national modifications.
See Presentation slides #36-38 for more information on licensing and the development of country modifications: Insights into the Next Revision: Like Texas, Everything is Bigger in ICD-11, Kathy Giannangelo, RHIA, CCS, CPHIMS, FHIMA, Texas Health Information Management Association.
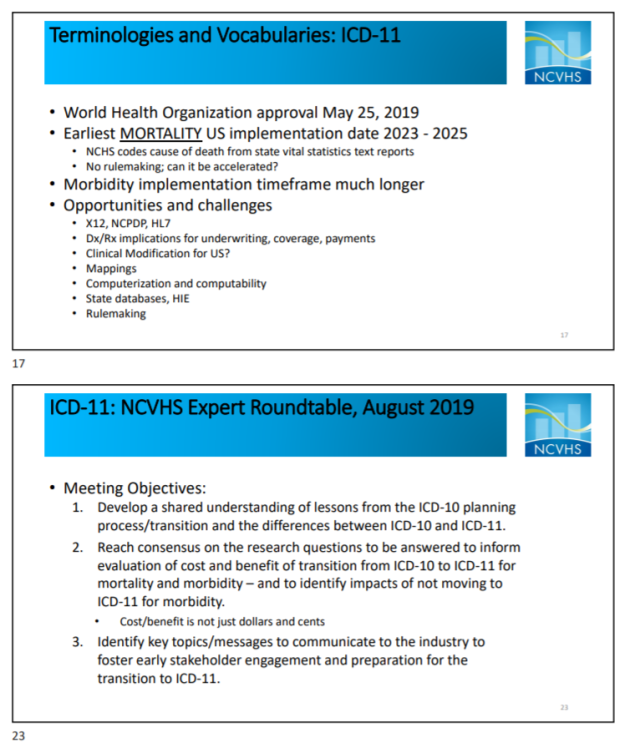
It would be premature to speculate when the U.S. might be ready to migrate to ICD-11 for mortality (cause of death reporting) and whether ICD-11 will be adequate as a morbidity classification system for U.S. use or whether NCHS will need to develop a clinical modification, as it did for ICD-10.
It was put forward at the June 5-6, 2019 NCVHS meeting that the U.S. might potentially use ICD-11 unmodified if WHO were to incorporate some additional terms within the global ICD-11 edition.
NCVHS has initiated the process of planning for transition to ICD-11 at the federal level.
In February 2019, William W Stead, MD, Chair, NCVHS, sent a letter to the Secretary of Health and Human Services (HHS) recommending a simplified process for adopting future versions of ICD. The letter also recommended that HHS should invest now in an ICD-11 evaluation project and develop a plan to enable a smooth, transparent transition from ICD-10 to ICD-11 at the optimal time.
NCVHS meetings:
The U.S. National Committee on Vital and Health Statistics (NCVHS) serves as the statutory public advisory body to the Secretary of Health and Human Services for health data, statistics, privacy, and national health information policy and the Health Insurance Portability and Accountability Act (HIPAA).
A National Committee on Vital and Health Statistics Full Committee Meeting was held on June 5-6, 2019.
Agenda: Full Committee Meeting-June 5-6, 2019
Presentations were given for Agenda item: ICD-11 Project:

Recording Mp3: Full Committee Meeting – Day 1 June 5, 2019
Agenda item: ICD-11 Project: presentations and discussions starts 2hrs: 50 mins in from start; closes 5hrs 55mins from start.
Recording Mp3: Full Committee Meeting – Day 2 June 6, 2019
Meeting summaries, transcripts, presentation slides may be available later.
ICD-11 Expert Roundtable Meeting August 6-7, 2019
National Committee on Vital and Health Statistics Subcommittee on Standards held an
ICD-11 Evaluation Expert Roundtable Meeting on August 6-7, 2019.
Mp3 recordings of this two day NICD-11 Expert Roundtable meeting are now available:
Recording Mp3 ICD-11 Expert Roundtable Meeting – Day 1 August 6, 2019
Recording Mp3 ICD-11 Expert Roundtable Meeting – Day 2 August 7, 2019
Update
Transcript Day 1 – August 6, 2019
[165pp]
Transcript Day 2 – August 7, 2019
[129pp]
These transcripts of the ICD-11 Roundtable two day meeting are 165 and 129 pages long and the files have only recently been posted on the NCVHS site.
I have not had time to review these yet, but they are essential reading for industry and public stakeholders in the U.S.’s potential adoption of ICD-11 or NCHS/CDC’s potential development of a clinical modification of ICD-11.
Federal Register notice of meeting:
PDF: https://www.govinfo.gov/content/pkg/FR-2019-07-08/pdf/2019-14375.pdf
Presentation slides may be available later.
Update:
A preliminary summary of the August 6-7, 2019 ICD-11 Roundtable meeting has now been posted by NCVHS:
International Classification of Diseases,
Eleventh Revision (ICD-11) Expert Roundtable
Publication Date: October 10, 2019 PDF: Preliminary Meeting Summary
Key Points for planned Letter to Secretary, HHS:

Appendix E: Final Research Questions [Will be inserted by Expert Roundtable group when final]
Appendix F: ICD-11 Communications Plan [Will be inserted by Expert Roundtable group when final]
Coding industry reports:
AHIMA Participates in ICD-11 Expert Roundtable summary by Sue Bowman, MJ, RHIA, CCS, FAHIMA, Aug 28, 2019
US gets the ball rolling on ICD-11 AAPC, August 16, 2019
1 WHO Group Discusses ICD-11 Transition Planning report by Sue Bowman, MJ, RHIA, CCS, FAHIMA for Journal of AHIMA (American Health Information Management Association)
2 Presentation: Status on ICD-11: The WHO Launch National Committee on Vital and Health Statistics, July 18, 2018, Donna Pickett, Chief, Classifications and Public Health Data Standards, Head, Collaborating Center for the WHO-FIC in North America; Robert N. Anderson, PhD Chief, Mortality Statistics Branch Division of Vital Statistics
WHO-FIC Africa
WHO-FIC Africa Collaborating Centre has said:
ICD-10 is the current standard for Morbidity (cause of illness) and Mortality (cause of death (COD) coding.
The ongoing implementation and maintenance of ICD-10 for mortality and morbidity coding remain a core focus of the WHO-FIC Collaborating Centre (African region). Following the release of ICD-11 MMS in June 2018, there will be increasing focus on ICD-11 in the work plan of the collaborating centre. Inputs to the development of ICD-11 are essential to ensure that the classification meets regional needs.
WHO-FIC Africa News: WHO on the Implementation of ICD-11, November 2018:
WHO-FIC collaborators met in Pretoria (South Africa) on 7 November 2018, discussing the implications for implementing ICD-11 and ICHI. We linked up with Nenad Kostanjsek from WHO (Geneva), who shared his thoughts about the preparation for implementation of ICD-11.
Other member states
This table from the eHealth DSI Semantic Knowledge Base project compiles information provided from a number of member states on their use of ICD (or a modification of ICD) and their plans regarding potential future implementation of ICD-11. Information provided by: Austria, Belgium, Croatia, Cyprus, Czech Republic, Estonia, France, Germany, Greece, Hungary, Ireland, Italy, Luxenbourg, Malta, Netherlands, Portugal, Slovenia and Spain.
Table: Current status of the use of ICD by eHDSI deploying countries (2018)
Resources:
ICD-11: The 11th Revision of the International Classification of Diseases – Site maintained by eHealth DSI Semantic Community providing resources for ICD-10, ICD-11, ICD derivative classifications and other classification and terminology systems
Insights into the Next Revision: Like Texas, Everything is Bigger in ICD-11, Kathy Giannangelo, RHIA, CCS, CPHIMS, FHIMA, Texas Health Information Management Association

