WHO retires “Benign” from “Benign myalgic encephalomyelitis” for final ICD-10 release
February 20, 2020
Post #357 Shortlink: https://wp.me/pKrrB-56g
In my report for the December edition of the ME Global Chronicle, I set out how the G93.3 terms:
Postviral fatigue syndrome
Benign myalgic encephalomyelitis
Chronic fatigue syndrome
are classified in the World Health Organization’s international version of ICD-10 and how these terms have been classified for ICD-11.
I have an update on ICD-10 and it’s good news!
In January, the WHO released ICD-10 Version: 2019. With ICD-11 on the horizon, this release will be the final update for the WHO’s international version of ICD-10, apart from corrections and exceptional additions.
In March 2016, a representative from the Canadian Institute for Health Information submitted a request and supporting rationale to the ICD-10 Update and Revision Committee (URC) for removal of the prefix “Benign” from “Benign myalgic encephalomyelitis”.
This request for a change was approved by the URC in September 2016 for implementation in the next release.
For ICD-10 Version: 2019, the G93.3 Tabular List inclusion term is now Myalgic encephalomyelitis.
(The term, “Benign myalgic encephalomyelitis” has been retained as an Index term.)
View the revised listing for the G93.3 codes on the ICD-10 Browser, here: https://icd.who.int/browse10/2019/en#/G93.3 or in the screenshot, below.
Note that for ICD-10, Chronic fatigue syndrome is not included in the Tabular List but is included in Volume 3: Index, where it is coded to the G93.3 Postviral fatigue syndrome concept title term.
For ICD-11, the WHO has retained Postviral fatigue syndrome as the concept title term in Chapter 08: Diseases of the nervous system under parent: Other disorders of the nervous system. The new code for ICD-11 is: 8E49.
Benign myalgic encephalomyelitis and Chronic fatigue syndrome are both specified as inclusion terms under Postviral fatigue syndrome in ICD-11’s equivalent to the Tabular List and take the 8E49 code. A number of historical and alternative terms are retained as index terms and all 14 index terms are coded to 8E49.
This is how the G93.3 terms are classified for ICD-10 Version: 2019:
Image 1: ICD-10 Browser Version: 2019, Accessed February 20, 2020: https://icd.who.int/browse10/2019/en#/G93.3
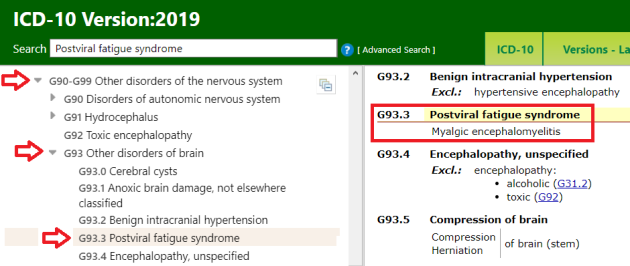
Image location: https://dxrevisionwatch.files.wordpress.com/2020/02/meicd1019.png
The WHO expects Member States to be using the most recent release of ICD-10. But countries will implement the ICD-10 Version: 2019 release according to their own schedules.
NHS England and ICD-10:
NHS England currently uses ICD-10 Version: 2016. I have contacted NHS Digital’s classifications lead to establish whether NHS Digital intends to implement Version: 2019 or may be considering skipping the new release in preference to implementing ICD-11, at some point in the future.
If there is no mechanism for incorporating selected changes in a new release into earlier versions, NHS England might not be able to absorb this change into the version it is using.
Will this change be absorbed automatically for ICD-11?
This revision for the final release of ICD-10 sets a precedent for the national modifications of ICD-10, for example, the U.S. ICD-10-CM and Canadian ICD-10-CA, but also for ICD-11.
Proposals submitted in March 2017 by Chapman & Dimmock, and by the IACFS/ME for removing “Benign” from “Benign myalgic encephalomyelitis” were rejected by the WHO in early 2019.
In February, I submitted a new proposal for removal of the “Benign” prefix for ICD-11 citing the URC’s 2016 decision and the implementation of that decision for the final release of ICD-10.
You can read a copy of my new proposal and rationale here: http://bit.ly/BenignICD11
I have updated the PDF included in my report in the December edition of the ME Global Chronicle to reflect this change:
Download the PDF of my updated report here:
Update on the classification of PVFS, ME and CFS for ICD-11 Report One | November 2019 | v3 18/02/20
An edited version of this report is scheduled for publication in the March edition of the ME Global Chronicle.





