What’s new in the ICD-11 Alpha drafting platform? (CFS, PVFS, ME)
April 10, 2012
What’s new in the ICD-11 Alpha drafting platform? (CFS, PVFS, ME)
Post #157 Shortlink: http://wp.me/pKrrB-22h
Screenshot: ICD-11 Alpha Browser Foundation view selected, logged in at April 10, 2012:
Chapter 6: Diseases of the nervous system
http://apps.who.int/classifications/icd11/browse/f/en#/http%3a%2f%2fwho.int%2ficd%23G93.3
Apr 09 – 11:02 UTC
ICD-11 Beta drafting platform to launch in May?
As reported in previous posts, according to the timeline, the ICD-11 Beta drafting platform is supposed to be launching this May.
ICD-11 Revision Steering Group has yet to announce whether the Beta platform remains on target for a May release and if so, on what date it will be launched – so I cannot give you a date yet.
Like the Alpha Drafting Browser, the Beta drafting platform will be a work in progress – not a final Beta draft. The final Beta isn’t scheduled until 2014, after the ICD-11 field trials have been undertaken.
When it does launch, the Beta platform is intended to be accessible to professionals and the public for viewing.
Registered or logged in users will have greater access to content and will be able to interact with the platform to read comments, comment on proposals and make suggestions, as part of the ongoing drafting process.
In the meantime, the publicly viewable version of the Alpha drafting platform (known as the ICD-11 Alpha Browser) can still be accessed here:
http://apps.who.int/classifications/icd11/browse/f/en
The various ICD-11 Revision Topic Advisory Groups are carrying out their draft preparation work on a separate, more complex multi-author drafting platform that is accessible only to WHO and ICD Revision personnel.
Alpha drafting platform
As before, the publicly viewable version of the Alpha Browser should be viewed with the following caveats in mind:
the Alpha draft is a work in progress; it is incomplete; it may contain errors and omissions; it is in a state of flux and updated daily; textual content, codes and “Sorting labels” are subject to change as chapters are reorganized and content populated; the content has not been approved by Topic Advisory Groups, Revision Steering Group or WHO.
It is possible to register, or sign into the platform using existing accounts with several third party account providers such as Google, Yahoo and myOpenID, for increased access and functionality. Once signed in, Comments and Questions can be read and PDFs of the drafts of the top level linearizations can be downloaded from the Linearization tab.
See the Alpha Browser User Guide for information on how the Alpha Browser functions:
http://apps.who.int/classifications/icd11/browse/Help/en
The ICD-11 “Content Model”
ICD-11 will be available in both print and online versions and unlike most chapters of ICD-10, will include descriptive content for ICD terms.
For the online version of ICD-11, all ICD entities will include a definition and a number of additional key descriptive fields – between 7 and 13 pre-defined parameters, populated according to a common “Content Model” (Content Model Reference Guide January 2011).
For example, ICD entity Title, Definition, Synonyms, Narrower Terms, Exclusions, Body Site, Body System, Signs and Symptoms, Causal Mechanisms, and possibly Diagnostic Criteria for some entities.*
*According to the iCAT User Google Group message board, these fields may have been revised since the January 2011 Content Model Reference Guide was published; Content Model parameters in the Beta draft may therefore differ from those currently displaying in the public Alpha drafting platform.
The print version will use a concise version of Definition due to space constraints.
In the Alpha Browser, not all these Content Model parameters display in the Foundation and Linearization views and not all of the parameters that have been listed for individual entities have had their draft text added yet, as some chapters are more advanced for the population of proposed content than others.
So the Alpha draft is still very patchy and many entities have no Definition and little or no other proposed content filled in.
With no “Category Discussion Notes” or “Change history” pop-up windows visible in the public version of the Alpha, the viewer cannot determine the rationales behind the reorganization of terms and hierarchies within the various chapters.
Chapter location and hierarchy for CFS, PVFS and (Benign) ME in ICD-11
I have been reporting since June 2010 that the proposals for ICD-11 Alpha Draft, as far as one could determine, appeared to be:
1] That a change of hierarchy had been recorded in a “Category Discussion Note”, dated May 1, 2010, between ICD-10 Title term “Postviral fatigue syndrome” and “Chronic fatigue syndrome”. (“Category Discussion Notes” and “Change History” pop-ups did display in the earlier iCAT version of the Alpha drafting platform.)
You can view a screenshot from June 2010 of that “Change history” record here:
https://dxrevisionwatch.com/wp-content/uploads/2010/06/change-history-gj92-cfs.png
The Definition field on the “Chronic fatigue syndrome” description panel in the current Alpha Browser is currently blank but in June 2010, the Definition had stood as in this contemporaneous screenshot:
https://dxrevisionwatch.com/wp-content/uploads/2010/05/2icatgj92cfsdef.png
2] That “Chronic fatigue syndrome” had been designated as an ICD-11 Title term within ICD-11 Chapter 6: Diseases of the nervous system, with the capacity for a Definition and up to 10 additional descriptive parameters.
3] That “Benign myalgic encephalomyelitis” had been specified as an Inclusion term to ICD-11 Title term “Chronic fatigue syndrome” but that the relationships between the three terms, PVFS, (B) ME and CFS had yet to be specified, as in this screenshot from June 2010:
https://dxrevisionwatch.com/wp-content/uploads/2010/05/2icatgj92cfsterms.png
What is currently showing in the Chapter 6 Foundation Component?
It isn’t possible to bring up a discrete ICD Title listing for either “Benign myalgic encephalomyelitis” or “Postviral fatigue syndrome” in either the Foundation Component or the Linearization.
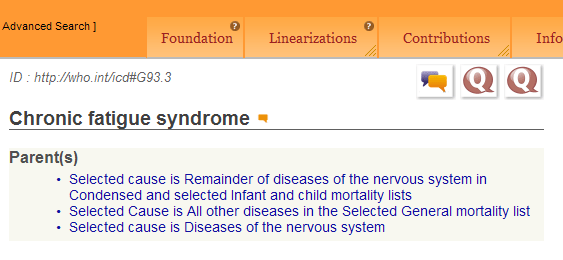
In the Foundation view only, for Chapter 6: Diseases of the nervous system, “Chronic fatigue syndrome” is listed as a Title term with the ICD-10 legacy ID “ID:http://who.int/icd#G93.3”;
the Definition field is currently blank;
a list of terms has recently been added under “Synonyms”;
one term has recently been added under “Narrower Terms”.
(Note: there is a small asterisk at the end of term “Benign myalgic encephalomyelitis” which is listed at the top of the “Synonyms” list. The asterisk “Hover text” reads “This term is an inclusion term in the linearizations.”)
If you want to view the listing directly on the Browser site (note the “Comment” and “Questions” icons which open up pop-up windows next to terms for reading/commenting won’t display unless you have already registered and logged in) go here:
ICD-11 Alpha Browser Foundation view:
http://apps.who.int/classifications/icd11/browse/f/en#/http%3a%2f%2fwho.int%2ficd%23G93.3
Chronic fatigue syndrome
Parent(s)
Selected cause is Remainder of diseases of the nervous system in Condensed and selected Infant and child mortality lists
Selected Cause is All other diseases in the Selected General mortality list
Selected cause is Diseases of the nervous systemDefinition
This entity does not have a definition at the moment.
Synonyms
Benign myalgic encephalomyelitis * [Ed: Hover text over asterisk reads: “This term is an inclusion term in the linearizations.”]
akureyri
akureyri disease
cfs – chronic fatigue syndrome
chronic fatigue syndrome nos [Ed: from current proposals for ICD-10-CM, Chapter 18, R53.82]
chronic fatigue, unspecified [Ed: from current proposals for ICD-10-CM, Chapter 18, R53.82]
epidemic neuromyasthenia
iceland disease
icelandic disease
me – myalgic encephalomyelitis
myalgic encephalomyelitis
myalgic encephalomyelitis syndrome
postviral fatigue syndrome
pvfs – postviral fatigue syndromeNarrower Terms
neuromyasthenia
Body Site
Entire brain (body structure)
Brain structure (body structure)Causal Mechanisms
Virus (organism)
What’s new in Chapter 5: Mental and behavioural disorders?
As reported in Dx Revision Watch post: http://wp.me/pKrrB-1Vx, the category “Somatoform Disorders” in Chapter 5, Mental and behavioural disorders is currently renamed to “BODILY DISTRESS DISORDERS”, under which currently sit three new child categories:
5M0 Mild bodily distress disorder
5M1 Moderate bodily distress disorder
5M2 Severe bodily distress disorder.
Chapter 5 Linearization view:
http://apps.who.int/classifications/icd11/browse/l-m/en#/http%3a%2f%2fwho.int%2ficd%23F45
Chapter 5 Foundation view:
http://apps.who.int/classifications/icd11/browse/f/en#/http%3a%2f%2fwho.int%2ficd%23F45
(Click on the little grey arrows to display the child categories):
Child categories to parent “BODILY DISTRESS DISORDERS”:
BODILY DISTRESS DISORDERS
5M0 Mild bodily distress disorder
5M1 Moderate bodily distress disorder
5M2 Severe bodily distress disorder
5M3 Somatization disorder
5M4 Undifferentiated somatoform disorder
5M5 Somatoform autonomic dysfunction
5M6 Persistent somatoform pain disorder
> 5M6.0 Persistent somatoform pain disorder
> 5M6.1 Chronic pain disorder with somatic and psycological [sic] factors
5M7 Other somatoform disorders
5M8 Somatoform disorder, unspecified
None of these three new (proposed) categories have had any Definitions or other textual content added to the description panels on the right hand side of the Alpha Browser page since I first reported this change in February.
It is still not possible to determine what disorders ICD-11 intends might be captured by these three new (proposed) terms, should ICD-11 Revision Steering Group and WHO classification experts consider these terms to be valid constructs and approve their progression through to the Beta draft.
Because no “Change Notes” or “Change history” pop-up windows display in this version of the Alpha Drafting browser, it is not possible to determine:
whether ICD-11 is proposing to introduce three new terms – 5M0 Mild bodily distress disorder; 5M1 Moderate bodily distress disorder; 5M2 Severe bodily distress disorder, in addition to retaining existing ICD-10 terms, 5M3 thru 5M8;
how ICD Revision intends to define these (proposed) new terms at 5M0, 5M1, 5M2;
how these three (proposed) new terms would relate to the existing ICD-10 “Somatoform Disorders” categories which remain listed as child categories to “BODILY DISTRESS DISORDERS” (apart from “Hypochondriacal disorder” [ICD-10: F45.2], which is now listed as “5H0.5 Illness Anxiety Disorder” in the ICD-11 Alpha Draft).
(See Page 1 and 2 of my report: “Bodily Distress Disorders” to replace “Somatoform Disorders” for ICD-11?: http://wp.me/pKrrB-1Vx )
References:
ICD-11 Revision: http://www.who.int/classifications/icd/revision/en/
ICD-11 Alpha Browser User Guide: http://www.who.int/classifications/icd/revision/caveat/en/index.html
Alpha Browser Foundation view: http://apps.who.int/classifications/icd11/browse/f/en#
Alpha Browser Linearization view: http://apps.who.int/classifications/icd11/browse/l-m/en#
“Bodily Distress Disorders” to replace “Somatoform Disorders” for ICD-11?: http://wp.me/pKrrB-1Vx