HHS announces Final Rule on ICD-10-CM compliance date
August 25, 2012
HHS announces Final Rule on ICD-10-CM compliance date
Post #202 Shortlink: http://wp.me/pKrrB-2uk
Update at August 26:
HHS Announces: ICD-10 Delayed One Year
The American Health Information Management Association (AHIMA) | August 24, 2012
…and finally…
Yesterday, August 24, Department of Health and Human Services (HHS) Secretary Kathleen Sebelius announced a final rule to delay compliance for adopting ICD-10-CM and ICD-10-PCS (ICD-10) code sets to October 1, 2014.
“The rule also makes final a one-year proposed delay – from Oct. 1, 2013, to Oct. 1, 2014– in the compliance date for use of new codes that classify diseases and health problems.”
http://www.hhs.gov/news/press/2012pres/08/20120824e.html
News Release
FOR IMMEDIATE RELEASE
August 24, 2012 Contact: U.S. Department of Health & Human Services
202-690-6343
New health care standards to save up to $6 billion
Today, Department of Health and Human Services (HHS) Secretary Kathleen Sebelius announced a final rule that will save time and money for physicians and other health care providers by establishing a unique health plan identifier (HPID). The rule is one of a series of changes required by the Affordable Care Act to cut red tape in the health care system and will save up to $6 billion over ten years.
“These new standards are a part of our efforts to help providers and health plans spend less time filling out paperwork and more time seeing their patients,” Secretary Sebelius said.
Currently, when a health care provider bills a health plan, that plan may use a wide range of different identifiers that do not have a standard format. As a result, health care providers run into a number of time-consuming problems, such as misrouting of transactions, rejection of transactions due to insurance identification errors, and difficulty determining patient eligibility. The change announced today will greatly simplify these processes.
The rule also makes final a one-year proposed delay – from Oct. 1, 2013, to Oct. 1, 2014– in the compliance date for use of new codes that classify diseases and health problems. These code sets, known as the International Classification of Diseases, 10th Edition diagnosis and procedure codes, or ICD-10, will include codes for new procedures and diagnoses that improve the quality of information available for quality improvement and payment purposes.
The rule announced today is the fourth administrative simplification regulation issued by HHS under the health reform law:
On July 8, 2011, HHS adopted operating rules for two electronic health care transactions to make it easier for health care providers to determine whether a patient is eligible for coverage and the status of a health care claim submitted to a health insurer. The rules will save up to $12 billion over ten years.
On Jan. 10, 2012, HHS adopted standards for the health care electronic funds transfers (EFT) and remittance advice transaction between health plans and health care providers. The standards will save up to $4.6 billion over ten years.
On Aug. 10, 2012, HHS published an IFC that adopted operating rules for the health care EFT and electronic remittance advice transaction. The operating rules will save up to $4.5 billion over ten years.
More information on the final rule is available in a fact sheet at http://www.cms.gov/apps/media/fact_sheets.asp
The final rule may be viewed at www.ofr.gov/inspection.aspx
###
Note: All HHS press releases, fact sheets and other press materials are available at http://www.hhs.gov/news
You can follow HHS on Twitter @HHSgov and sign up for HHS Email Updates.
Last revised: August 24, 2012
CENTERS FOR MEDICARE & MEDICAID SERVICES
RULES
Administrative Simplification:
Adoption of Standard for Unique Health Plan Identifier; Addition to National Provider Identifier Requirements, etc.
2012-21238
[CMS 0040 F; Filed: 08/24/12 at 12:00pm; Publication Date: 9/5/2012]
http://www.ofr.gov/OFRUpload/OFRData/2012-21238_PI.pdf
or download here: ![]() 2012-21238_PI
2012-21238_PI
Extract:
(3) ICD-10-CM and ICD-10-PCS Code Sets
In the January 16, 2009 Federal Register (74 FR 3328), HHS published a final rule in which the Secretary of HHS (the Secretary) adopted the ICD-10-CM and ICD-10-PCS (ICD-10) code sets as the HIPAA standards to replace the previously adopted International Classification of Diseases, 9th Revision, Clinical Modification, Volumes 1 and 2 (diagnoses), and 3 (procedures) including the Official ICD–9–CM Guidelines for Coding and Reporting. The compliance date set by the final rule was October 1, 2013.
Since that time, some provider groups have expressed strong concern about their ability to meet the October 1, 2013 compliance date and the serious claims payment issues that might ensue if they do not meet the date. Some providers’ concerns about being able to meet the ICD-10 compliance date are based, in part, on difficulties they had meeting the compliance deadline for the adopted Associated Standard Committee’s (ASC) X12 Version 5010 standards (Version 5010) for electronic health care transactions. Compliance with Version 5010 and ICD-10 by all covered entities is essential to a smooth transition to the updated medical data code sets, as the failure of any one industry segment to achieve compliance would negatively affect all other industry segments and result in returned claims and provider payment delays. We believe the change in the compliance date for ICD-10 gives covered health care providers and other covered entities more time to prepare and fully test their systems to ensure a smooth and coordinated transition by all covered entities.
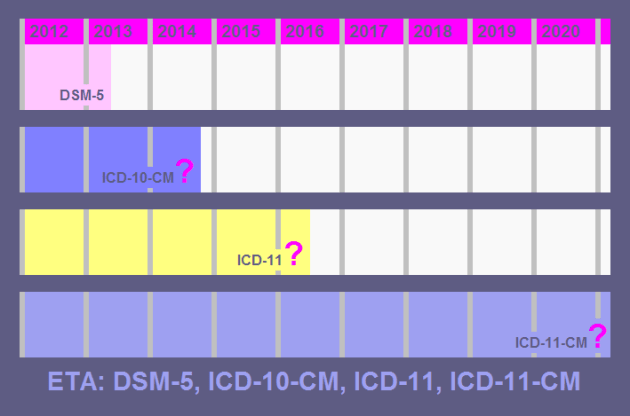

Reminder: Comment period on ICD-10-CM proposed delay ends May 17
April 25, 2012 by meagenda
Reminder: Comment period on ICD-10-CM proposed delay ends May 17
Post #159 Shortlink: http://wp.me/pKrrB-23H
On April 9, the US Department of Health and Human Services issued a proposed rule calling for a one year delay in the ICD-10-CM/PCS compliance deadline.
The proposed rule would postpone the compliance date by which providers and industry have to adopt ICD-10-CM by one year, from October 1, 2013 to October 1, 2014.
The proposed rule was published in the Federal Register on April 17, followed by a 30 day period during which CMS will take comments.
Comments should be submitted to HHS no later than 5:00 pm ET on May 17, 2012.
Proposed Rule
More information on the proposed rule is available from this CMS fact sheet
HHS PROPOSES ONE-YEAR DELAY OF ICD-10 COMPLIANCE DATE (CMS-0040-P)
Submitting comment
Submitting comment by post:
Centers for Medicare & Medicaid Services
Department of Health and Human Services
Attention: CMS–0040–P
P.O. Box 8013
Baltimore, MD 21244–8013
Submitting comment online:
Go to the Federal Regulations website, here:
http://www.regulations.gov/#!documentDetail;D=CMS-2012-0043-0001
Hit the Submit a Comment button, top right of web page
http://www.regulations.gov/#!submitComment;D=CMS-2012-0043-0001
For delivery by hand see the Alternate Ways to Comment pop up, top right of Submit a Comment page.
Related material
Press release: April 9, 2012
Summary Proposal Rule
This proposed rule would implement section 1104 of the Patient Protection and Affordable Care Act (hereinafter referred to as the Affordable Care Act) by establishing new requirements for administrative transactions that would improve the utility of the existing Health Insurance Portability and Accountability Act of 1996 (HIPAA) transactions and reduce administrative burden and costs. It proposes the adoption of the standard for a national unique health plan identifier (HPID) and requirements or provisions for the implementation of the HPID. This rule also proposes the adoption of a data element that will serve as an other entity identifier (OEID), an identifier for entities that are not health plans, health care providers, or “individuals,” that need to be identified in standard transactions. This proposed rule would also specify the circumstances under which an organization covered health care provider must require certain noncovered individual health care providers who are prescribers to obtain and disclose an NPI. Finally, this rule proposes to change the compliance date for the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) for diagnosis coding, including the Official ICD-10-CM Guidelines for Coding and Reporting, and the International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) for inpatient hospital procedure coding, including the Official ICD-10-PCS Guidelines for Coding and Reporting, from October 1, 2013 to October 1, 2014.
Share this:
Filed under Clinical Modification, CMS, HHS, ICD revision process, ICD-10, ICD-10-CM, ICD-10-CM compliance, ICD-10-PCS Tagged with CMS Public Affairs, comment period, hhs, icd coding, ICD-10 delay, icd-10-cm, ICD-10-CM compliance, ICD-10-CM final rule, proposed rule, sibelius