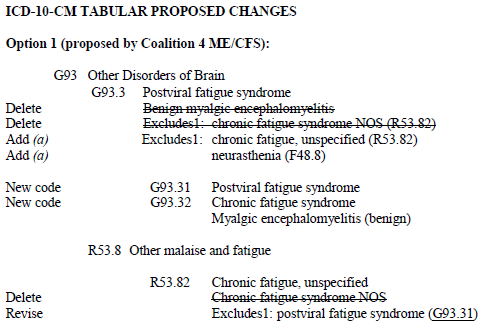
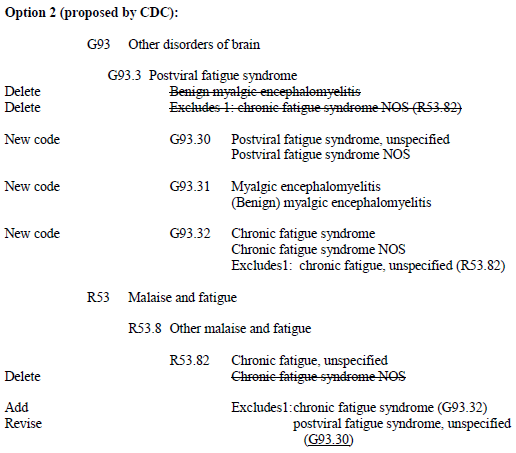
October 31, 2012
by admindxrw
APA finally posts DSM-5 Field Trials online and DSM-5 Round up
Post #206 Shortlink: http://wp.me/pKrrB-2vu
Three papers discussing the results of the DSM-5 field trials were posted online yesterday by the American Journal of Psychiatry. The papers describe the methods and results of the 23 diagnoses assessed during the field trials.
APA failed to publish field trial results during the life of the third and final public review and comment period.
Access to the abstracts is free but you will need subscriber or institution access for the full PDFs or cough up $$ for the papers. ($35 per paper for 24 hours’ access. Why have these reports not been published on the DSM-5 Development website? Many classes of stakeholder will be disenfranchised.)
The article states that criteria were tested in October 2010 through February 2012 by 279 clinicians at 11 U.S. and Canadian academic centers. A second set of data from small group practices and private practices is expected to be reported early next year (that is, after the finalized draft has gone to the publishers).
Proposed criteria are still under review and won’t be finalized until approved by APA Board of Trustees.
DSM-5 draft proposals for criteria and categories as issued for the third and final stakeholder review can be read here on the DSM-5 Development website.
Note that the draft is now frozen and criteria sets and manual texts subject to embargo until publication of the DSM-5 manual. Any revisions made by the Task Force and Work Groups since the third iteration was released in May, this year, won’t be reflected on the DSM-5 Development website.
Published yesterday in the American Journal of Psychiatry and at Psychiatry Online:
Tuesday, October 30, 2012
Full text of article:
DSM-5 Field Trials Posted Online by AJP
http://alert.psychiatricnews.org/2012/10/dsm-5-field-trials-posted-online-by-ajp.html
+++
Article 1 | October 30, 2012
Abstract: http://psychiatryonline.org/article.aspx?articleid=1387935
DSM-5 Field Trials in the United States and Canada, Part I: Study Design, Sampling Strategy, Implementation, and Analytic Approaches
Diana E. Clarke, Ph.D., M.Sc.; William E. Narrow, M.D., M.P.H.; Darrel A. Regier, M.D., M.P.H.; S. Janet Kuramoto, Ph.D., M.H.S.; David J. Kupfer, M.D.; Emily A. Kuhl, Ph.D.; Lisa Greiner, M.S.S.A.; Helena C. Kraemer, Ph.D.
Am J Psychiatry 2012;:. 10.1176/appi.ajp.2012.12070998
PDF for those with subscriber access: http://ajp.psychiatryonline.org/data/Journals/AJP/0/appi.ajp.2012.12070998.pdf
+++
Article 2 | October 30, 2012
Abstract: http://psychiatryonline.org/article.aspx?articleid=1387906
DSM-5 Field Trials in the United States and Canada, Part II: Test-Retest Reliability of Selected Categorical Diagnoses
Darrel A. Regier, M.D., M.P.H.; William E. Narrow, M.D., M.P.H.; Diana E. Clarke, Ph.D., M.Sc.; Helena C. Kraemer, Ph.D.; S. Janet Kuramoto, Ph.D., M.H.S.; Emily A. Kuhl, Ph.D.; David J. Kupfer, M.D.
Am J Psychiatry 2012;:. 10.1176/appi.ajp.2012.12070999
PDF for those with subscriber access:
http://ajp.psychiatryonline.org/data/Journals/AJP/0/appi.ajp.2012.12070999.pdf
+++
Article 3 | October 30, 2012
Abstract: http://psychiatryonline.org/article.aspx?articleid=1387907
DSM-5 Field Trials in the United States and Canada, Part III: Development and Reliability Testing of a Cross-Cutting Symptom Assessment for DSM-5
William E. Narrow, M.D., M.P.H.; Diana E. Clarke, Ph.D., M.Sc.; S. Janet Kuramoto, Ph.D., M.H.S.; Helena C. Kraemer, Ph.D.; David J. Kupfer, M.D.; Lisa Greiner, M.S.S.A.; Darrel A. Regier, M.D., M.P.H.
Am J Psychiatry 2012;:. 10.1176/appi.ajp.2012.12071000
PDF for those with subscriber access:
http://ajp.psychiatryonline.org/data/Journals/AJP/0/appi.ajp.2012.12071000.pdf
Commentaries:
DSM5 in Distress
The DSM’s impact on mental health practice and research.
by Allen Frances, M.D.
DSM 5 Field Trials Discredits APA
You can’t turn a sow’s ear into a silk purse.
…According to the authors, 14 of the 23 disorders had “very good” or “good” reliability; 6 had questionable, but ‘acceptable’ levels; and just three had “unacceptable” rates. Sounds okay until you look at the actual data and discover that the cheerful words used by the DSM 5 leaders simply don’t fit their extremely disappointing results. The paper is a classic example of Orwellian ‘newspeak’…
Allen Frances, M.D. | August 30, 2012
Read full article here
Also on Huffington Post
+++
1 Boring Old Man
finally…
1 Boring Old Man | October 30, 2012
Well, they finally published the results of the DSM-5 Field Trials. Here are the links to the abstracts and the main table of kappa values to look over…
DSM-5 Round up
Public Lecture St Mary’s College of Maryland
http://www.smcm.edu/calendar/events/index.php?com=detail&eID=2317
DSM-V: Social, Political, and Ethical Implications
November 2
3:00 PM – 5:00 PM
Cole Cinema, Campus Center
This presentation will describe the DSM-V, scheduled for publication in May 2013, and the controversy surrounding its development. Dr. Ancis will provide an overview of the newly proposed classification system and diagnoses.
It is imperative that those involved in using the DSM-V, or potentially impacted by the DSM, be duly informed. Questions associated with the DSM-V revision process; the empirical bases of proposed changes; social, legal, and political implications; and ethical and cultural considerations will be addressed.
Dr. Ancis will describe her involvement in a number of initiatives related to DSM-V proposals, including those of the Association of Women in Psychology and Counselors for Social Justice. She will also review concerns of major mental health organizations worldwide, such as the American Psychological Association, the American Counseling Association, and the British Psychological Society, and related divisions.
Dr. Ancis is currently a Professor of Counseling and Psychological Services at Georgia State University. She earned her Bachelors, Masters, and Ph.D from the University at Albany, State University of New York. Her major areas of interest are multicultural competency training, diversity attitudes, race and gender issues, education and career development, and legal system experiences.
Event Contact Info
Janet Kosarych-Coy
Email: jmkosarychcoy@smcm.edu
Phone: 2408954283
Website: Click to Visit
Location: Cole Cinema, Campus Center
18952 E. Fisher Rd
St. Mary’s City, MD 20686
Categories:
Current Students
Faculty/Staff
Lecture
Open to the Public
Psychology
Psychology Today
Side Effects
From quirky to serious, trends in psychology and psychiatry
The Tranquilizer Trap The scandal over benzodiazepines gets different emphasis in the UK and U.S.
Published on October 3, 2012 by Christopher Lane, Ph.D. in Side Effects
Anti-DSM Sentiment Rises in France Why French psychiatrists and psychoanalysts are opposed to the diagnostic manual (French Stop DSM-5 Campaign)
Published on September 28, 2012 by Christopher Lane, Ph.D. in Side Effects
New York Times
Report Sees Less Impact in New Autism Definition
By BENEDICT CAREY | Published: October 2, 2012
Proposed changes to the official diagnosis of autism will not reduce the proportion of children found to have it as steeply as many have feared, scientists reported on Tuesday, in an analysis that contradicts several previous studies…
Medscape
Medscape Medical News > Psychiatry
Controversial New Diagnosis in DSM-5 May Be Faulty
Pam Harrison | October 17, 2012
Attenuated psychosis syndrome (APS), a new and controversial diagnosis for potential inclusion in the upcoming Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), is questionable, new research suggests…
DSM-5 and Employment Law
In September, Douglas Hass (Franczet Radelet) published an article Could the American Psychiatric Association Cause You Headaches? The Dangerous Interaction between the DSM-5 and Employment Law:
Abstract:
http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2153268
Since its first publication in 1952, the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM) has long served not only as the primary reference for mental health disorders for medical practitioners, but also as a primary authority for the legal community…
Full text in PDF format: Hass
Research Article
http://onlinelibrary.wiley.com/doi/10.1002/da.22012/abstract
Research Article
The Effect of Draft DSM-V Criteria on Posttraumatic Stress Disorder Prevalence
Patrick S. Calhoun Ph.D.1,2,3,*,
Jeffrey S. Hertzberg B.A.3,
Angela C. Kirby M.S.3,
Michelle F. Dennis B.A.2,
Lauren P. Hair M.S.3,
Eric A. Dedert Ph.D.1,2,3,
Jean C. Beckham Ph.D.1,2,3
Article first published online: 26 OCT 2012
DOI: 10.1002/da.22012
© 2012 Wiley Periodicals, Inc.
Journal of Psychosomatic Research
November 2012 Issue, Journal of Psychosomatic Research
http://www.jpsychores.com/current
Issue: Vol 73 | No. 5 | November 2012 | Pages 325-400
http://www.jpsychores.com/article/S0022-3999(12)00225-5/abstract
Predictive validity and clinical utility of DSM-5 Somatic Symptom Disorder – Comparison with DSM-IV somatoform disorders and additional criteria for consideration
Katharina Voigt
Affiliations
Department of Psychosomatic Medicine and Psychotherapy, University Medical Center Hamburg-Eppendorf and Schön Klinik Hamburg-Eilbek, Hamburg, Germany
Corresponding author at: Department of Psychosomatic Medicine and Psychotherapy, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany. Tel.: +49 40 7410 54408; fax: +49 40 7410 54975.
Eileen Wollburg
Affiliations
Schön Klinik Bad Bramstedt, Bad Bramstedt, Germany
Nina Weinmann
Affiliations
Schön Klinik Bad Bramstedt, Bad Bramstedt, Germany
Annabel Herzog
Affiliations
Department of Psychosomatic Medicine and Psychotherapy, University Medical Center Hamburg-Eppendorf and Schön Klinik Hamburg-Eilbek, Hamburg, Germany
Björn Meyer
Affiliations
GAIA AG, Hamburg, Germany
Gernot Langs
Affiliations
Schön Klinik Bad Bramstedt, Bad Bramstedt, Germany
Bernd Löwe
Affiliations
Department of Psychosomatic Medicine and Psychotherapy, University Medical Center Hamburg-Eppendorf and Schön Klinik Hamburg-Eilbek, Hamburg, Germany
Received 3 July 2012; received in revised form 29 August 2012; accepted 30 August 2012; published online 24 September 2012.
Abstract
Objective
Major changes to the diagnostic category of somatoform disorders are being proposed for DSM-5. The effect of e.g. the inclusion of psychological criteria (criterion B) on prevalence, predictive validity, and clinical utility of “Somatic Symptom Disorder” (SSD) remains unclear. A prospective study was conducted to compare current and new diagnostic approaches.
Methods
In a sample of N=456 psychosomatic inpatients (61% female, mean age=44.8±10.4years) diagnosed with somatoform, depressive and anxiety disorders, we investigated the current DSM-5 proposal (SSD) plus potential psychological criteria, somatic symptom severity, and health-related quality of life at admission and discharge.
Results
N=259 patients were diagnosed with DSM-IV somatoform disorder (56.8%). With a threshold of 6 on the Whiteley Index to assess psychological criteria, the diagnosis of SSD was similarly frequent (51.8%, N=230). However, SSD was a more frequent diagnosis when we employed the recommended threshold of one subcriterion of criterion B. Patients diagnosed with only SSD but not with DSM-IV somatoform disorder showed greater psychological impairment. Both diagnoses similarly predicted physical functioning at discharge. Bodily weakness and somatic and psychological attributions at admission were among significant predictors of physical functioning at discharge. Reduction of health anxiety, bodily weakness, and body scanning significantly predicted an improvement of physical functioning.
Conclusions
Psychological symptoms enhance predictive validity and clinical utility of DSM-5 Somatic Symptom Disorder compared to DSM-IV somatoform disorders. The SSD diagnosis identifies more psychologically impaired patients than its DSM-IV precursor. The currently suggested diagnostic threshold for criterion B might increase the disorder’s prevalence.
Keywords: Somatoform disorder, Diagnosis, Diagnostic and Statistical Manual of Mental Disorders, Classification of diseases, Validation studies as topic
Ed: Note: Between publication of the second iteration of the DSM-5 draft proposals for public review and publication of the third set of draft proposals, the SSD “B type criteria” were reduced from the requirement to meet at least two from the “B type” criteria to at least one [1].
1] http://www.dsm5.org/ProposedRevision/Pages/proposedrevision.aspx?rid=368
Somatic Symptom Disorder Criteria