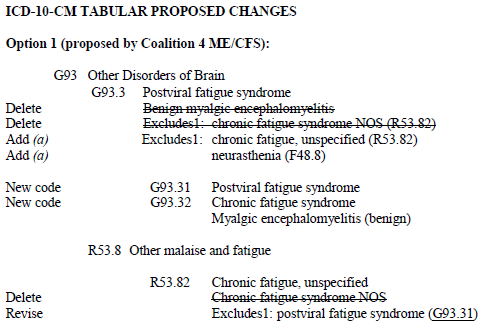
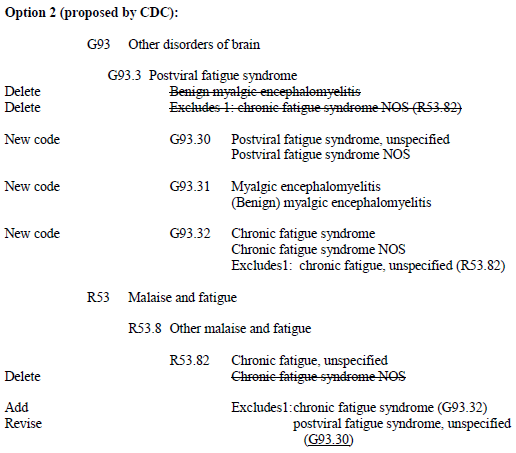
ICD-11 Round up: April #1
April 24, 2013
ICD-11 Round up: April #1
Post #239 Shortlink: http://wp.me/pKrrB-2Qy
[PMID 23583019]
The Lancet, Early Online Publication, 11 April 2013
doi:10.1016/S0140-6736(12)62191-6
Maercker A, Brewin CR, Bryant RA, Cloitre M, Reed GM, Ommeren MV, Humayun A, Jones LM, Kagee SA, Llosa AE, Rousseau C, Somasundaram DJ, Souza R, Suzuki Y, Weissbecker I, Wessely SC, First MB, Saxena S.
Mental disorders specifically associated with stress are exceptional in needing external events to have caused psychiatric symptoms for a diagnosis to be made. The specialty of stress-associated disorders is characterised by lively debates, including about the extent to which human suffering should be medicalised, 1 and the purported overuse of the diagnosis of post-traumatic stress disorder (PTSD). 2 Most common mental disorders are potentiated or exacerbated by stress and childhood adversity…
Contributors
AM, CRB, RAB, MC, GMR, MvO, SW, MBF, and SS were the core writing group. AH, LJ, SAK, AEL, CR, DS, RS, YS, and IW discussed the text and gave feedback to the core writing group.
Conflicts of interest
AM, CRB, RAB, MC, AH, LJ, SAK, CR, DS, SCW, and YS are members of the WHO ICD-11 Working Group on the Classification of Disorders Specifically Associated with Stress, reporting to the WHO International Advisory Group for the Revision of ICD-10 Mental and Behavioural Disorders. GMR, MvO, and SS are members of the WHO Secretariat, Department of Mental Health and Substance Abuse. AEL, RS, IW, and MBF are special invitees to Working Group meetings. However, the views expressed in this article are those of the authors and, except as specifically noted, do not represent the official policies or positions of the International Advisory Group or WHO.
[Subscription required for full paper. A PDF may be available on authors’ personal websites or academic websites.]
According to CDC’s, Donna Picket, as reported by AHIMA (American Health Information Management Association), “ICD-11 would likely not be ready for implementation in the US until after 2020.”
AHIMA
Update: ICD-11 on Track For 2015
Melanie Endicott | AHIMA & ICD-10 & ICD-10/CAC Summit | April 23, 2013
While the United States is preparing to implement ICD-10-CM/PCS on October 1, 2014, the World Health Organization (WHO) is anticipating a 2015 release of ICD-11. Taking into account the need to then clinically modify the WHO version, ICD-11 would likely not be ready for implementation in the US until after 2020. Donna Pickett, MPH, RHIA, medical systems administrator at Centers for Disease Control and Prevention/National Center for Health Statistics, delivered an update on the progress of ICD-11 development in Monday’s presentation “ICD-11 Update” at the 2013 AHIMA ICD-10-CM/PCS and Computer-Assisted Coding Summit, taking place in Baltimore, MD this week... Read on
Go here to view ICD-11 Beta drafting platform public version
http://www.who.int/classifications/icd/revision/betaexpectations/en/
ICD-11 Beta: Expectations, Concerns and Known Issues
Information for Beta Participants
ICD-11 Beta Phase started on 14 May 2012. The objective is to have a final ICD-11 version by 2015. This announcement clarifies that ICD-11 Beta version is not final, and will be enhanced by input from multiple stakeholders during the beta phase, which will last 3 years.
Caveats
Problems and Issues
Concerns and Criticisms etc
http://www.who.int/classifications/icd/revision/en/index.html
Revision
Participate in ICD Revision
Video invitation to participate
Frequently Asked Questions About ICD-11
ICD Information Sheet
ICD Revision Information Notes
Register to become involved
Timelines
Content Model
Definitions etc
Presentation | T Bedirhan Üstün
ICD Revision Summary presentation: Quality and Safety Topic Advisory Group meeting, New York, April 2-3, 2013.
ICD11 Quality and Safety TAG 2013 Presentation | Slideshare
According to this presentation, by WHO’s Bedirhan Üstün, all ICD-11 Topic Advisory Groups (TAGs) have finished their editing of the structure. A good deal of work remains for the population of content, in accordance with the ICD-11 Content Model, across all chapters and on compatibility of linearizations across primary care, specialty and detailed research versions.
Presentation [PDF Format, no PP viewer required]
Revising the ICD Definition of Intellectual Disability: Implications and Recommendations | March 19, 2013
Intellectual Disability’s Long Journey: George Jesien, Ph.D., Executive Director, Association for University Centers on Disabilities (AUCD)
Intellectual Disability and the Revision of ICD-10 Mental and Behavioural Disorders: Geoffrey M. Reed, Department of Mental Health and Substance Abuse, WHO
AAIDD Proposed Recommendations for ICD-11: Marc J. Tassé, Nisonger Center – UCEDD, The Ohio State University, Webinar
On Slides 17 and 18, Classification System Most Used and Classification Most Used by Country, graphics for data from WPA-WHO Survey of Practicing Psychiatrists* on global use of ICD-10, ICD-8/9, DSM-IV and Other diagnostic system(s).
*World Psychiatry. 2011 Jun;10(2):118-31.
The WPA-WHO Global Survey of Psychiatrists’ Attitudes Towards Mental Disorders Classification.
Reed GM, Mendonça Correia J, Esparza P, Saxena S, Maj M.
Medscape
Schizophrenia Bulletin
Schizophr Bull.2012;38(5):895-898.
Status of Psychotic Disorders in ICD-11
Wolfgang Gaebel
Abstract and full report